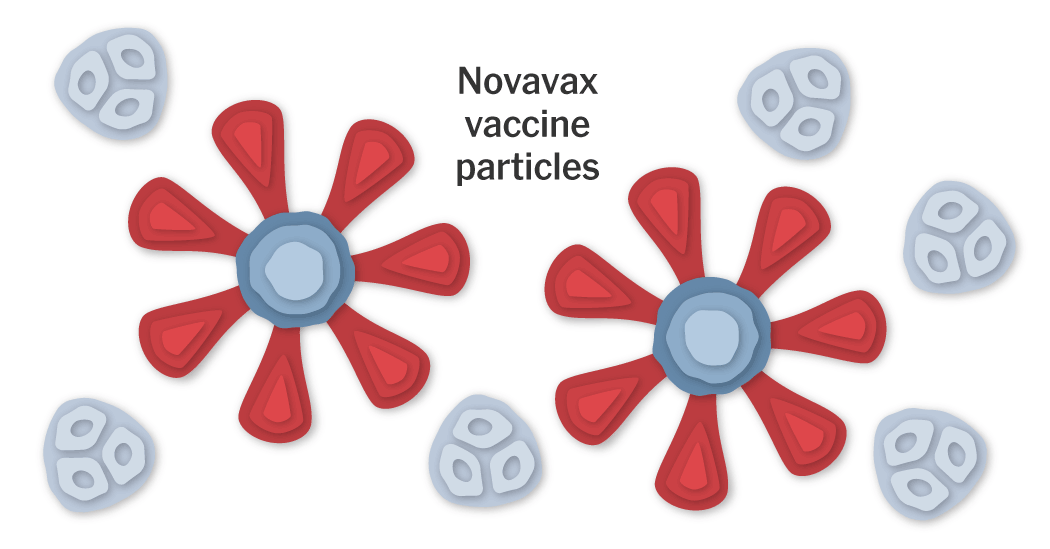
The Maryland-based company Novavax has developed a protein-based coronavirus vaccine called NVX-CoV2373. The vaccine produced strikingly high levels of antibodies in early clinical trials. In September, the vaccine entered a Phase 3 clinical trial in the United Kingdom, and another one in the United States at the end of December. Those trials will show whether the vaccine is safe and effective.
Coronavirus Proteins
The SARS-CoV-2 virus is studded with proteins that it uses to enter human cells. These so-called spike proteins make a tempting target for potential vaccines and treatments.


The Novavax vaccine works by teaching the immune system to make antibodies to the spike protein.
Growing Spike Proteins
To create their vaccine, Novavax researchers started with a modified spike gene. They inserted the gene into a different virus, called a baculovirus, and allowed it to infect insect cells. The infected cells produced spike proteins that spontaneously joined together to form spikes, as they do on the surface of the coronavirus.

Three spike
proteins combine

Three spike
proteins combine

Three spike
proteins combine

Three spike
proteins combine

Three spike
proteins combine

Three spike
proteins combine
A similar method of growing and harvesting virus proteins is already used to make licensed vaccines for diseases including influenza and HPV.
Building Nanoparticles
The researchers harvested the spike proteins from the insect cells and assembled them into nanoparticles. While the nanoparticles mimicked the molecular structure of the coronavirus, they could not replicate or cause Covid-19.

Nanoparticle
studded with
spikes

Nanoparticle
studded with
spikes

Nanoparticle
studded with
spikes
Presenting the Spike
The vaccine is injected into the muscles of the arm. Each injection includes many spike nanoparticles, along with a compound extracted from the soapbark tree. The compound attracts immune cells to the site of the injection and causes them to respond more strongly to the nanoparticles.


Immunity-priming
compound

Immunity-priming
compound
Spotting the Intruder
Immune cells called antigen-presenting cells encounter the vaccine nanoparticles and take them up.

Presenting
spike protein
fragments

Presenting
spike protein
fragments

Presenting
spike protein
fragments
An antigen-presenting cell tears apart the spike proteins and displays some of their fragments on its surface. A so-called helper T cell may detect the fragments. If a fragment fits into one of its surface proteins, the T cell becomes activated. Now it can recruit other immune cells to respond to the vaccine.
Making Antibodies
Another type of immune cell, called a B cell, may also encounter the vaccine nanoparticles. B cells have surface proteins in a huge variety of shapes, and a few might have the right shape to latch onto a spike protein. If a B cell does latch on, it can pull the vaccine particle inside and present spike protein fragments on its surface.
If a helper T cell activated against the spike protein latches onto one of these fragments, it activates the B cell. Now the B cell proliferates and pours out antibodies that have the same shape as its surface proteins.

Matching
surface proteins

Matching
surface proteins

Matching
surface proteins

Matching
surface proteins

Matching
surface proteins

Matching
surface proteins

Matching
surface
proteins

Matching
surface
proteins

Matching
surface
proteins

Matching
surface proteins

Matching
surface proteins

Matching
surface proteins
Stopping the Coronavirus
If vaccinated people are later exposed to the coronavirus, their antibodies can lock onto the spike proteins. The coronavirus cannot enter cells, and the infection is blocked.
Killing Infected Cells
The Novavax vaccine can also trigger another kind of protection by destroying infected cells. When a coronavirus invades, infected cells put fragments of its spike protein on their surface. Antigen-presenting cells can activate a type of immune cell called a killer T cell. It can recognize coronavirus-infected cells and destroy them before they have a chance to produce new viruses.

Presenting a
spike protein
fragment
Beginning
to kill the
infected cell

Presenting a
spike protein
fragment
Beginning
to kill the
infected cell

Presenting a
spike protein
fragment
Beginning
to kill the
infected cell

Presenting a
spike protein
fragment
Beginning to kill
the infected cell

Presenting a
spike protein
fragment
Beginning to kill
the infected cell

Presenting a
spike protein
fragment
Beginning to kill
the infected cell

Presenting a
spike protein
fragment
Beginning to kill
the infected cell

Presenting a
spike protein
fragment
Beginning to kill
the infected cell

Presenting a
spike protein
fragment
Beginning to kill
the infected cell

Presenting a
spike protein
fragment
Beginning to kill
the infected cell

Presenting a
spike protein
fragment
Beginning to kill
the infected cell

Presenting a
spike protein
fragment
Beginning to kill
the infected cell
Remembering the Virus
Novavax’s vaccine would be easier to distribute and store than the vaccines from Pfizer-BioNTech and Moderna. While those vaccines have to be kept frozen, NVX-CoV2373 can stay stable for up to three months in a refrigerator. But if the vaccine does turn out to be effective, scientists won’t know for sure how long it provides protection.

Second dose
21 days later

Second dose
21 days later

Second dose
21 days later
If it works like protein-based vaccines for other diseases, it may create a group of special cells called memory B cells and memory T cells. These cells will retain information about the coronavirus for years or even decades, enabling a quick counterattack in response to a new infection.
Vaccine Timeline
January, 2020 Novavax begins work on a coronavirus vaccine.
![]()
A screen showing protein structures at a Novavax lab in Maryland.Andrew Caballero-Reynolds/Agence France-Presse
May Novavax launches clinical trials for their vaccine.
July The U.S. government awards Novavax $1.6 billion to support the vaccine’s clinical trials and manufacturing.
August Novavax launched a Phase 2 trial on 2,900 people in South Africa.
![]()
Preparing an injection in Johannesburg, South Africa.Joao Silva/The New York Times
September Novavax launches a Phase 3 trial with up to 15,000 volunteers in the United Kingdom. The trial is expected to deliver results in early 2021.
Dec. 28 Novavax launches a Phase 3 trial with 30,000 people in the United States. The trial had been delayed because of problems with manufacturing the doses required for the study.
2021 If its clinical trials succeed, Novavax expects to deliver 100 million doses for use in the United States in 2021.
Sources: National Center for Biotechnology Information; Nature Reviews Immunology; Science; Maria Elena Bottazzi, Baylor College of Medicine.
Tracking the Coronavirus
[ad_2]
Source link