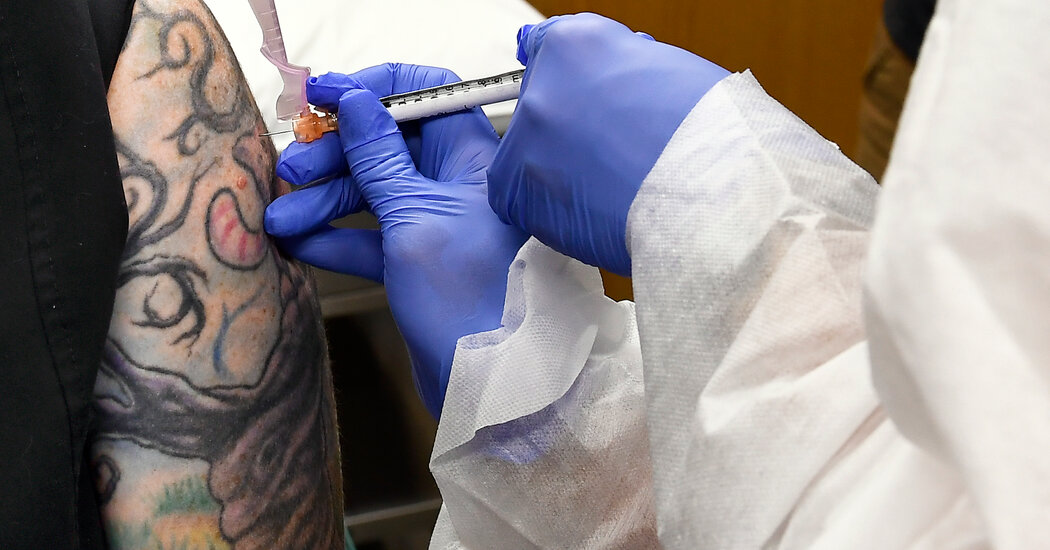
A coalition of leading public health experts urged the Food and Drug Administration on Wednesday night to conduct full safety and efficacy reviews of potential coronavirus vaccines before making the products widely available to the public.
In a letter signed by nearly 400 experts in infectious diseases, vaccines and other medical specialties, the group called on Dr. Stephen Hahn, the F.D.A. commissioner, to be forthcoming about the agency’s deliberations over whether to approve any new vaccine, in order to gain the public’s trust.
“We must be able to explain to the public what we know and what we don’t know about these vaccines,” noted the letter, which was organized by the nonprofit Center for Science in the Public Interest. “For that to happen, we must be able to witness a transparent and rigorous F.D.A. approval process that is devoid of political considerations.”
More than 30 experimental coronavirus vaccines are in clinical trials, with several companies racing to have the first product in the United States ready by the end of the year. The federal government has promised more than $9 billion to companies for these efforts to date. But many people are highly skeptical of these new vaccines, and might refuse to get them.
“Collaborations between scientists, the pharmaceutical industry and the federal government may bring us to a remarkable and historic achievement,” the letter said. “But an effective vaccine will only be truly useful if a large proportion of the public is willing to take it.”
The signers included academic researchers and former government officials from around the country, including the former surgeon general Dr. Joycelyn Elders; the former F.D.A. chief Dr. Jane E. Henney; and Dr. Luciana Borio, the former director for medical and biodefense preparedness at the National Security Council.
In an effort to reassure the public, Dr. Hahn said recently that he would seek the advice of the F.D.A.’s Vaccines and Related Biological Products Advisory Committee, although he has not said when the group would meet or which vaccine candidates it would consider.
The F.D.A. declined to comment on the letter Wednesday evening.
Dr. Paul Offit, a professor at the University of Pennsylvania and a member of the F.D.A.’s vaccine advisory panel, was also among the signers. In an interview, Dr. Offit called the agency’s emergency authorization for hydroxychloroquine — a malaria drug that President Trump has promoted as a treatment for Covid-19, despite no evidence that it works — a “warning shot.” The authorization was later revoked after a review found that 100 Covid-19 patients who took the drug had serious heart problems, including 25 who died.
“I think the administration bent or imposed its will on the F.D.A.,” Dr. Offit said. “There’s a concern that this would happen here, too.”
The letter said that scientists carrying out vaccine trials should share the details of their Phase 3 trials, which test thousands of volunteers to see whether the products prevent coronavirus infections and whether they cause side effects. It’s critical, the letter said, that the F.D.A. not approve any product until Phase 3 data are complete. The group also requested that volunteers be monitored for unexpected side effects that occur after the trials.
The Coronavirus Outbreak ›
Frequently Asked Questions
Updated August 4, 2020
-
I have antibodies. Am I now immune?
- As of right now, that seems likely, for at least several months. There have been frightening accounts of people suffering what seems to be a second bout of Covid-19. But experts say these patients may have a drawn-out course of infection, with the virus taking a slow toll weeks to months after initial exposure. People infected with the coronavirus typically produce immune molecules called antibodies, which are protective proteins made in response to an infection. These antibodies may last in the body only two to three months, which may seem worrisome, but that’s perfectly normal after an acute infection subsides, said Dr. Michael Mina, an immunologist at Harvard University. It may be possible to get the coronavirus again, but it’s highly unlikely that it would be possible in a short window of time from initial infection or make people sicker the second time.
-
I’m a small-business owner. Can I get relief?
- The stimulus bills enacted in March offer help for the millions of American small businesses. Those eligible for aid are businesses and nonprofit organizations with fewer than 500 workers, including sole proprietorships, independent contractors and freelancers. Some larger companies in some industries are also eligible. The help being offered, which is being managed by the Small Business Administration, includes the Paycheck Protection Program and the Economic Injury Disaster Loan program. But lots of folks have not yet seen payouts. Even those who have received help are confused: The rules are draconian, and some are stuck sitting on money they don’t know how to use. Many small-business owners are getting less than they expected or not hearing anything at all.
-
What are my rights if I am worried about going back to work?
-
Should I refinance my mortgage?
- It could be a good idea, because mortgage rates have never been lower. Refinancing requests have pushed mortgage applications to some of the highest levels since 2008, so be prepared to get in line. But defaults are also up, so if you’re thinking about buying a home, be aware that some lenders have tightened their standards.
-
What is school going to look like in September?
- It is unlikely that many schools will return to a normal schedule this fall, requiring the grind of online learning, makeshift child care and stunted workdays to continue. California’s two largest public school districts — Los Angeles and San Diego — said on July 13, that instruction will be remote-only in the fall, citing concerns that surging coronavirus infections in their areas pose too dire a risk for students and teachers. Together, the two districts enroll some 825,000 students. They are the largest in the country so far to abandon plans for even a partial physical return to classrooms when they reopen in August. For other districts, the solution won’t be an all-or-nothing approach. Many systems, including the nation’s largest, New York City, are devising hybrid plans that involve spending some days in classrooms and other days online. There’s no national policy on this yet, so check with your municipal school system regularly to see what is happening in your community.
Dr. Offit said that the advisory board, which includes experts from academia, industry and government and makes much of its discussions public, could handle as many of the vaccine candidates as are ready to review.
“Typically they are two-day meetings,” he said. “We could make them longer than that. We can go through all the data.”
Dr. Rebekah Gee, chief executive of Louisiana State University health care services, who also signed the letter, said that research data on the vaccines must be made public.
“Everyone in our nation is anxiously awaiting a Covid-19 vaccine,” she said. “It’s important to public health to save lives, and for our economy, but we want to make sure that whatever is done is done in an open process that is devoid of political influence.”
[ad_2]
Source link