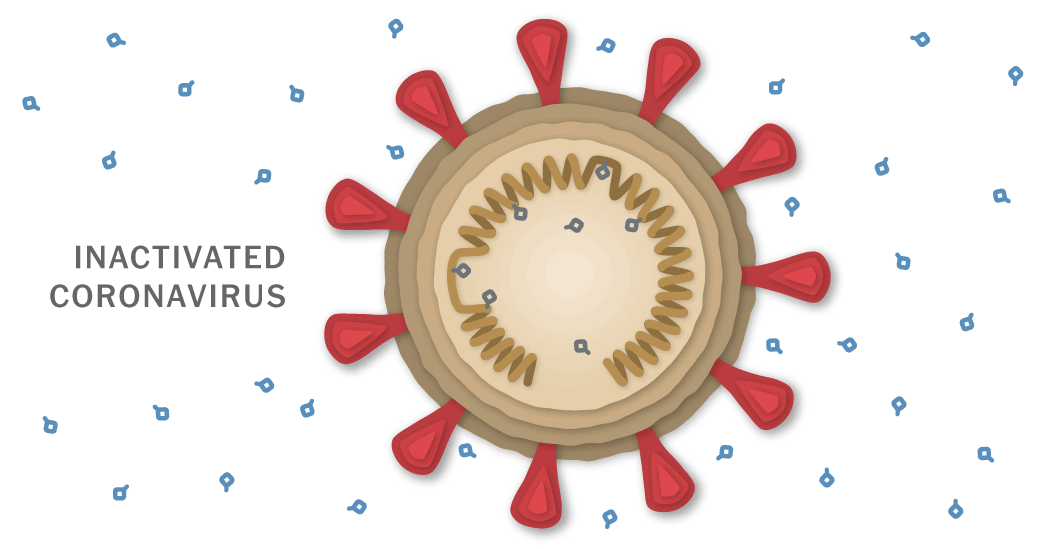
The private Chinese company Sinovac developed a coronavirus vaccine called CoronaVac. On Dec. 23, Brazilian researchers announced that the vaccine had an efficacy of over 50 percent. The complete details of Sinovac’s Phase 3 trials are expected in early January.
A Vaccine Made From Coronaviruses
CoronaVac works by teaching the immune system to make antibodies against the SARS-CoV-2 coronavirus. The antibodies attach to viral proteins, such as the so-called spike proteins that stud its surface.
To create CoronaVac, the Sinovac researchers started by obtaining samples of the coronavirus from patients in China, Britain, Italy, Spain and Switzerland. One sample from China eventually served as the basis for the vaccine.
Killing the Virus
The researchers grew large stocks of the coronavirus in monkey kidney cells. Then they doused the viruses with a chemical called beta-propiolactone. The compound disabled the coronaviruses by bonding to their genes. The inactivated coronaviruses could no longer replicate. But their proteins, including spike, remained intact.
The researchers then drew off the inactivated viruses and mixed them with a tiny amount of an aluminum-based compound called an adjuvant. Adjuvants stimulate the immune system to boost its response to a vaccine.
Inactivated viruses have been used for over a century. Jonas Salk used them to create his polio vaccine in the 1950s, and they’re the bases for vaccines against other diseases including rabies and hepatitis A.
Prompting an Immune Response
Because the coronaviruses in CoronaVac are dead, they can be injected into the arm without causing Covid-19. Once inside the body, some of the inactivated viruses are swallowed up by a type of immune cell called an antigen-presenting cell.
Presenting
virus protein
fragments
Presenting
virus protein
fragments
Presenting
virus protein
fragments
The antigen-presenting cell tears the coronavirus apart and displays some of its fragments on its surface. A so-called helper T cell may detect the fragment. If the fragment fits into one of its surface proteins, the T cell becomes activated and can help recruit other immune cells to respond to the vaccine.
Making Antibodies
Another type of immune cell, called a B cell, may also encounter the inactivated coronavirus. B cells have surface proteins in a huge variety of shapes, and a few might have the right shape to latch onto the coronavirus. When a B cell locks on, it can pull part or all of the virus inside and present coronavirus fragments on its surface.
A helper T cell activated against the coronavirus can latch onto the same fragment. When that happens, the B cell gets activated, too. It proliferates and pours out antibodies that have the same shape as their surface proteins.
Matching
surface proteins
Matching
surface proteins
Matching
surface proteins
Matching
surface proteins
Matching
surface proteins
Matching
surface proteins
Matching
surface
proteins
Matching
surface
proteins
Matching
surface
proteins
Matching
surface proteins
Matching
surface proteins
Matching
surface proteins
Stopping the Virus
Once vaccinated with CoronaVac, the immune system can respond to an infection of live coronaviruses. B cells produce antibodies that stick to the invaders. Antibodies that target the spike protein can prevent the virus from entering cells. Other kinds of antibodies may block the virus by other means.
Remembering the Virus
While CoronaVac can offer some protection against Covid-19, no one can yet say how long that protection lasts. It’s possible that the level of antibodies drops over the course of months. But the immune system also contains special cells called memory B cells that might retain information about the coronavirus for years or even decades.
Vaccine Timeline
January, 2020 Sinovac begins developing an inactivated vaccine against the coronavirus.
Sinovac engineers working with monkey kidney cells.Nicolas Asfouri/Agence France-Presse
June Phase 1/2 trials on 743 volunteers find no severe adverse effects.
July Sinovac launches a Phase 3 trial in Brazil, followed by others in Indonesia and Turkey. Reuters reports that the Chinese government gave the Sinovac vaccine emergency approval for limited use.
A dose of CoronaVac in Turkey.Emrah Gurel/Associated Press
October Authorities in the eastern Chinese city of Jiaxing announce they are giving CoronaVac to people in relatively high-risk jobs, including medical workers, port inspectors and public service personnel.
Oct. 19 Officials in Brazil say that Sinovac is the safest of five vaccines they are testing in Phase 3 trials.
November Sinovac publishes the details of its Phase 1/2 trial in a medical journal, showing a comparatively modest production of antibodies. Only a Phase 3 trial will demonstrate if that is enough to protect people from Covid-19.
Nov. 19 The Brazilian government announces that they paused the country’s Sinovac trial the previous month because of an adverse event. The details of the pause were murky, raising suspicions that politics were involved. Two days after the announcement, the trial was allowed to resume. The Brazilian trial has recorded enough cases of Covid-19 to let researchers determine Sinovac’s efficacy. They expect to release their results by Dec. 23.
Officials in Brazil hold boxes from a shipment of the vaccine.Alexandre Schneider/Getty Images
December Sinovac says it expects to manufacture 300 million doses in 2020 and increase capacity to an annual production of 600 million doses.
Dec. 23 Brazilian researchers announce that CoronaVac has an efficacy of over 50 percent.
Sources: National Center for Biotechnology Information; Science; The Lancet; Lynda Coughlan, University of Maryland School of Medicine; Jenna Guthmiller, University of Chicago.
Tracking the Coronavirus
[ad_2]
Source link