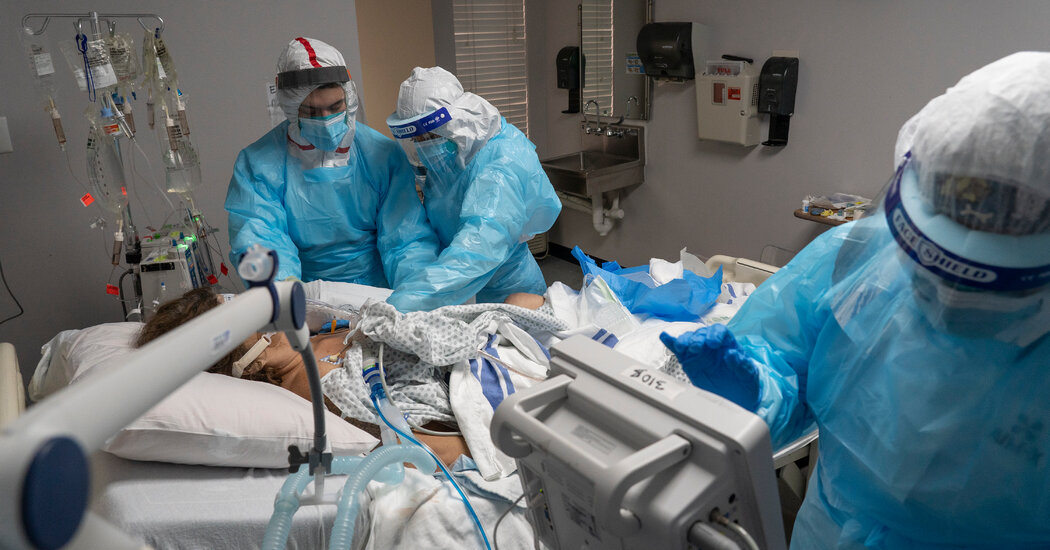
Adding an arthritis drug called baricitinib to Covid treatment regimens that include the antiviral drug remdesivir might shave a day or more off recovery times, especially for those who are seriously sick, according to a study published on Friday.
The findings of a government-sponsored clinical trial were made public more than three weeks after the Food and Drug Administration issued an emergency use authorization for the dual treatment. Earlier this month, some experts said they were uncomfortable deploying drugs without the opportunity to vet the underlying data supporting their performance. Last month, the World Health Organization also recommended against remdesivir as a treatment for Covid patients because evidence supporting its use was lacking.
Limited results earlier were announced via news releases, showing that hospitalized Covid patients treated with baricitinib and remdesivir recovered one day faster than those who had received remdesivir alone.
Some questioned adopting the combination treatment given baricitinib’s hefty price tag — which might be about $1,500 per patient — and also cited side effects like blood clots. Several doctors also wondered whether adding baricitinib would be worthwhile because steroids like dexamethasone were cheap and widely available. Both baricitinib and dexamethasone are thought to act by tamping down excessive inflammation, which drives many severe cases of Covid.
The new paper, published in the New England Journal of Medicine, adds some granularity to the findings, showing that certain subgroups of patients benefited from the addition of baricitinib far more than others. The trial enrolled more than 1,000 hospital patients with Covid, all of whom received remdesivir. People who were sick enough to require a high dose of supplemental oxygen or a non-invasive form of ventilation recovered eight days faster when baricitinib was included in their drug regimen.
In these groups, “I think the data clearly supports a role for baricitinib,” said Dr. Boghuma Kabisen Titanji, an infectious disease physician at Emory University who pioneered early studies of baricitinib against the coronavirus.
Dr. Titanji also noted that the data hinted that certain patients might be less likely to die or need a ventilator if they took baricitinib in addition to remdesivir. But these results, like the results that showed faster recovery times, weren’t uniform across the trial’s participants.
Dr. Lauren Henderson, a pediatric rheumatologist at Boston Children’s Hospital, said she was encouraged by the results and the prospect of having another option in the treatment arsenal against the coronavirus.
But she and several other experts added that they might still be inclined to default to dexamethasone as a treatment for severely sick Covid-19 patients who needed respiratory support.
Dexamethasone, unlike baricitinib, has been shown in studies to curb mortality in seriously ill Covid patients. It’s also inexpensive and easy to obtain, while baricitinib is considered more of a specialty drug, potentially posing supply chain obstacles, said Dr. Erin McCreary, an infectious disease pharmacist at the University of Pittsburgh.
Emerging Treatments for Covid-19
Words to Know About Covid-19 Treatment
Confused by the terms used about how to treat Covid-19? Let us help:
-
- ACE-2: A protein that sits on the surface of certain types of human cells. The coronavirus has to bind to ACE-2 in order to enter cells.
- Adverse event: A health problem that crops up in volunteers in a clinical trial of a vaccine or a drug. An adverse event isn’t always caused by the treatment tested in the trial.
- Antibody: A protein produced by the immune system that can attach to a pathogen such as the coronavirus and stop it from infecting cells.
- Antiviral drug: A drug that interferes with a virus’s ability to replicate inside cells. The first approved drug for Covid-19 in the United States, remdesivir, is an antiviral.
- Approval, licensure and emergency use authorization: Drugs, vaccines and medical devices cannot be sold in the United States without gaining approval from the Food and Drug Administration, also known as licensure. After a company submits the results of clinical trials to the F.D.A. for consideration, the agency decides whether the product is safe and effective, a process that generally takes many months. If the country is facing an emergency — like a pandemic — a company may apply instead for an emergency use authorization, which can be granted considerably faster.
- Compassionate use: A term used to describe treatments that are given to seriously ill people despite not yet being approved by the Food and Drug Administration for that use.
- Cytokine storm: A hyperactive response from the immune system that can lead to massive amounts of inflammation and tissue damage. Cytokine storms may be responsible for many of the severe cases of Covid-19, and a number of researchers are testing drugs that might be able to quiet them.
- Interferon: A molecule made by the immune system. Certain types of interferons can unleash inflammation in the body, while others tamp it down. Yet other types can spur cells to strengthen their defenses against viruses. Researchers are exploring whether treatments of synthetic interferons can help people fight off the coronavirus.
- Monoclonal antibodies: Monoclonal antibodies, created in a laboratory, mimic the natural antibodies produced by the immune system. A number of companies have developed these treatments for Covid-19. President Trump received Regeneron’s antibody treatment shortly after he was diagnosed with the disease.
- Phase 1, 2, and 3 trials: Clinical trials typically take place in three stages. Phase 1 trials usually involve a few dozen people and are designed to observe whether a vaccine or drug is safe. Phase 2 trials, involving hundreds of people, allow researchers to try out different doses and gather more measurements about the vaccine’s effects on the immune system. Phase 3 trials, involving thousands or tens of thousands of volunteers, determine the safety and efficacy of the vaccine or drug by waiting to see how many people are protected from the disease it’s designed to fight.
- Placebo: A substance that has no therapeutic effect, often used in a clinical trial. To see if a vaccine can prevent Covid-19, for example, researchers may inject the vaccine into half of their volunteers, while the other half get a placebo of salt water. They can then compare how many people in each group get infected.
- Post-market surveillance: The monitoring that takes place after a vaccine or drug has been approved and is regularly prescribed by doctors. This typically confirms that the treatment is safe. On rare occasions, it detects side effects in certain groups of people that were missed during clinical trials.
- Preclinical research: Studies that take place before the start of a clinical trial, typically involving experiments where a treatment is tested on cells or in animals.
- Trial protocol: A series of procedures to be carried out during a clinical trial.
- Retrospective study: A study that analyzes data collected in the past to assess how effective a treatment is. Retrospective studies can offer useful insights, but are not as definitive as randomized clinical trials.
- Spike protein: A protein that sits on the surface of coronaviruses. The spike protein binds to the ACE-2 receptor on human cells using a region called the receptor-binding domain (R.B.D.). Once the protein attaches, the virus can enter the cell. Many vaccines and monoclonal antibody treatments are designed to attach to the spike.
- Standard of care: A treatment accepted by medical experts as a proper way to treat a certain type of disease. Once a standard of care emerges for a disease, any new experimental treatments are typically tested against it, rather than against a placebo.
Several experts pointed to another National Institutes of Health trial that will conduct a head-to-head comparison of two combination treatment regimens: one that puts hospitalized patients on remdesivir and baricitinib, and another that pairs remdesivir with dexamethasone. Dr. McCreary also noted the importance of studying patients who receive both baricitinib and dexamethasone “to determine if there is incremental benefit.”
Dr. Andre Kalil, an infectious disease physician at the University of Nebraska Medical Center and the lead researcher on the new paper, noted that while dexamethasone had already become a widely accepted treatment for Covid-19, the steroid still needed further study. He cited “a multitude of serious safety issues” with the drug that warranted some scrutiny.
Like other steroids, dexamethasone, which broadly blunts inflammation, can come with a host of unwanted side effects, including exacerbating conditions like diabetes or osteoporosis.
[ad_2]
Source link